Author: Mark Heinemann, Senior NIS Submissions Specialist, inVentiv Health Clinical
According to the provisions of article L.1453-1 CSP, pharmaceutical companies must now disclose the existence of agreements that they concluded with:
- Healthcare professionals,
- Associations of healthcare professionals,
- Students in medicine and odontology,
- Associations of patients,
- Health establishments,
- Foundations,
- Press organs for health professionals for all media (press, radio, TV or online communication);
- Medical prescription and deliverance software editors;
- Learned societies.
Each company must disclose the information for each agreement concluded.
Above a limit of 10 euros all taxes includes, the obligation of disclosure also applies to the advantages in nature or in money granted directly or indirectly by pharmaceutical companies to the above listed recipients. Consequently, all advantages granted to persons listed above that are above 10 euros will have to be disclosed.
The said information will be disclosed in French on a unique public website held by an authority to be created by an order of the Ministry of Health.
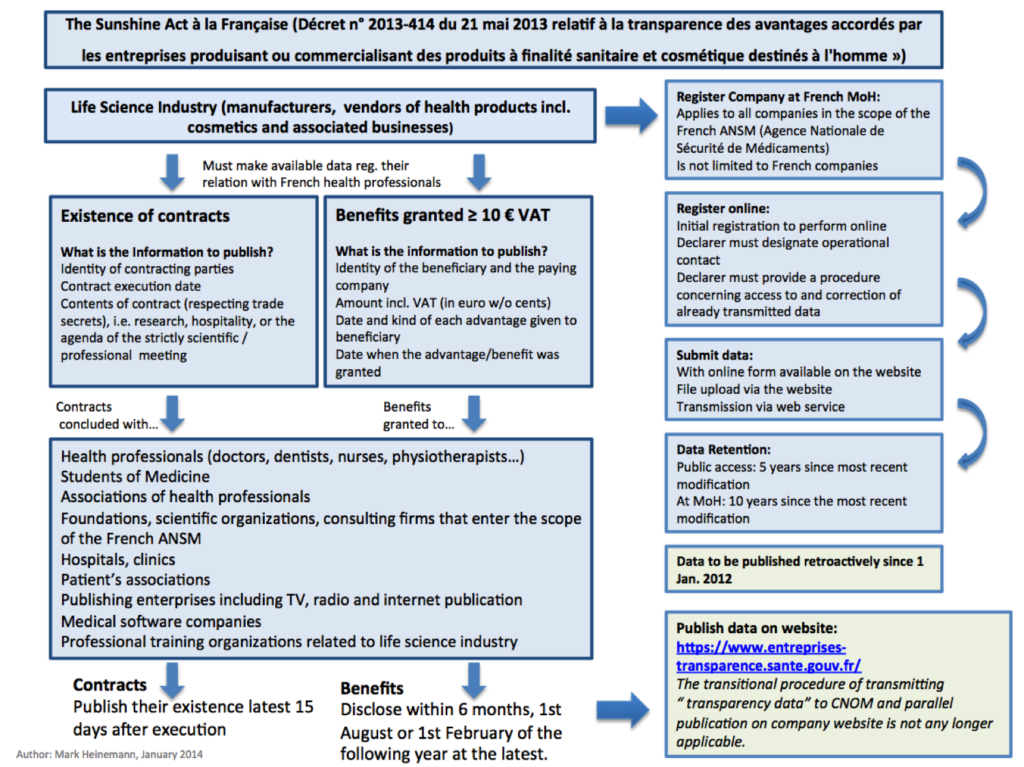
We have provided a summary and flowchart of these requirements below:
- Summary of the French Transparency Law incorporating changes of 09Jan.2013
- The Sunshine Act à la Française 15Jan13
